Sensitization
Sensitization is a term used to describe the precipitation of carbides at the grain boundaries of metals or alloys generally during high-temperature exposure. In most cases, carbides are formed due to depleted levels of chromium and other corrosion resistance elements and, because of this steel is in danger of intergranular corrosion (IGC) or intergranular stress corrosion cracking (SCC).
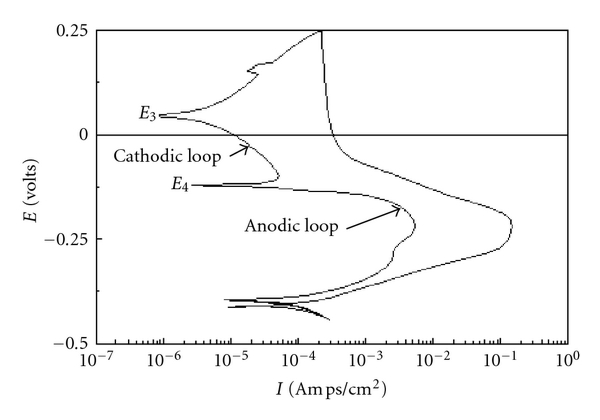
Calculation of Degree of Sensitization
By the electrochemical calculations
[(Ir/Ia)*100 = DoS %]
Where,
Ir = Reactivation Current
Ia = Activation Current
DoS % = percentage of sensitization
Preventions that can be taken for account –
1 Use the L grade steels i.e., low carbon grades like 309L, 316L instead of 309 or 316 so that less carbides will form results in lesser sensitization
2 Induce of corrosion resistance elements like Chromium (Cr), Molobium (Mo), Nickel (Ni), Titanium (Ti) and others, so that the resistance properties got better like by the introduction of Molobium, thermal stability of the alloy got better, introduction of Chromium will be highly resistant to corrosion and many more
3 The temperature elevation is directly proportion to the degradation of mechanical and surface properties of the metal (in most of the cases but exceptions are always there), for example if you give a metal thermal ageing treatment for let’s say 800 C then water quench it’s going to show degradation in Corrosion resistance as carbides are formed and they don’t allow the corrosion resistance elements so perform, hence lower the exposure of critical temperature range lesser would be the sensitization in the metal.
Time-Temperature-Sensitization Curves
One of the most popular curves for the analysis of the sensitization is by plotting the sensitization temperature and annealing time as a function of some measure sensitization factor.